What is the antigen rapid detection test?
What is an antigen test?
In response to the growing COVID-19 pandemic and shortages of laboratory-based molecular testing capabilities and reagents, several manufacturers of diagnostic tests have developed and started selling fast and easy-to-use devices to facilitate testing outside of the laboratory setting test. These COVID-19 Antigen Rapid Detection Test kits are based on detecting proteins from the COVID-19 virus in respiratory samples (e.g. sputum, throat swabs), or human antibodies in blood or serum due to infection.
The difference between antigen detection and nucleic acid detection
The principles of antigen detection and nucleic acid detection are different.
The nucleic acid detection of the new coronavirus uses the principle of molecular biology. Through reverse transcription, amplification and quantitative detection of the nucleic acid sequence of the pathogen, the virus detection sensitivity and specificity are high, and the cost is high.
Antigen detection uses the principle of immunology, which can be completed quickly in a non-laboratory environment. The sensitivity of antigen detection is about 75%, and the detection sensitivity and specificity are worse than nucleic acid detection.
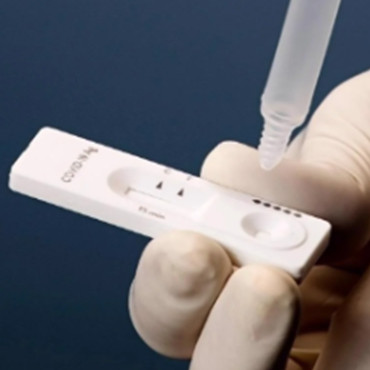
Can the results of the self-test using the antigen test kit replace the nucleic acid test results?
Cannot! We want to make it clear that the final result is to add antigen detection as a supplement on the basis of nucleic acid detection. Nucleic acid testing is still the basis for the diagnosis of new coronavirus infection.
Antigen detection is generally used in the acute infection period, that is, the detection of samples within 7 days of the onset of symptoms in the suspected population. Both positive and negative antigen results of suspected populations should undergo further nucleic acid testing. Positive results can be used for early triage and rapid management of suspected populations, but cannot be used as the basis for the diagnosis of new coronavirus infection.
Who are the antigen detection kits suitable for?
Persons who are undergoing home isolation, close contact and sub-close contact, entry isolation observation, closed and controlled areas, and control areas should be organized and managed by relevant management departments.
Introduction to the characteristics of rapid diagnostic tests based on antigen detection
A rapid diagnostic test (RDT) detects the presence of viral proteins (antigens) expressed by the COVID-19 virus in human respiratory samples. If a sufficient concentration of the target antigen is present in the sample, it will bind to specific antibodies immobilized on paper strips in a plastic casing and produce a visually detectable signal, usually within 30 minutes. The detected antigen is only expressed when the virus is actively replicating; therefore, such tests are best used to identify acute or early infection.
The effectiveness of the test depends on several factors, including the time of onset, the concentration of virus in the sample, the quality and handling of the sample taken from the person, and the precise formulation of the reagents in the kit. test suite. Based on experience with antigen-based RDTs for other respiratory diseases, such as influenza, where affected patients have concentrations of influenza virus in respiratory samples comparable to those seen in COVID-19, the sensitivity of these tests may range from 34% to 80% change.
Based on this information, such tests may miss half or more of the patients with COVID-19, depending on the group of patients tested. These hypotheses urgently require further study to see if they are accurate. In addition, if the antibodies on the test strip also recognize viral antigens other than COVID-19, such as antigens from the human coronavirus, the common cold. If any of the antigen detection tests in development or commercialization show sufficient performance, they may be used as triage tests to rapidly identify patients who are likely to have COVID-19.
Professional manufacturer of Nucleic Acid (DNA & RNA) Extraction and Analysis products COVID-19 Antigen Rapid Detection Test supplier
GENETURE is a group company,we own two factories: Ascend and Dianrun,to provide one stop solution of Nucleic Acid Extraction and Analysis,including solution for COVID-19. Geneture provides high quality and professional Nucleic Acid Extraction Reagents, Lab consumables, Real-time PCR consumables and test machines.
GENETURE main products including: Nucleic acid extraction or purification kit,Automatic nucleic acid extractor, PCR system, PCR kit, Magnetic beads, and lab consumables of 96 well deep plate,Magnetic rod comb,PCR tube,PCR plate,Pipette tips,centrifuge tubes.
If you have any questions or any need for COVID-19 Antigen Rapid Detection Test kit, you are welcome to contact GENETURE at any time.
Email: info@geneture.com
Mobile: +86 150 1002 8687
